NONTACT
Technical Specifications
PRODUCT DETAILS
- Flexible hand covering made of sturdy, impermeable silicone (proven to resist microbial growth)
- Pocket-sized accessory
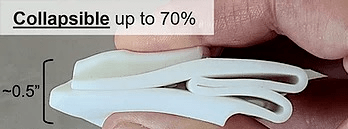
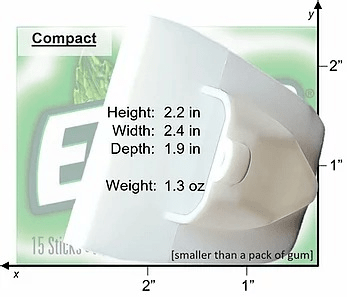
One-size-fits-all: Worn over thumb and 1-4 fingertips

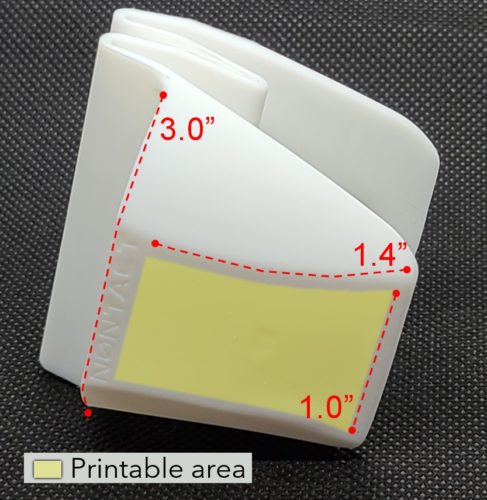
Customization / Branding
- NONTACT finger gloves available in all standard colors
- Logo printing available, flexible, and customized per Customer needs
- Lead time: 1-4 weeks, depending on design colors and unit volume

Use & Care instructions for current Customers:
Concerns over liability risk
- NONTACT finger gloves fall under “improvised PPE” which extends the same use authorization as conventional PPE for related applications (e.g., latex gloves)
- Our US-based manufacturing follows strict production & quality standards, and is FDA-approved for medical devices (though NONTACT is not intended for medical and other highly technical applications)
- HHS declared in March 2020 that businesses producing, distributing, or administering use of PPE as COVID-19 “countermeasures” have tort immunity under the PREP Act, and cannot be sued under state or federal law
- Note that these statements should not be taken as legal advice; for a confirmatory interpretation of the HHS declaration and the PREP Act, please refer to this well-explained summary by The National Law Review